Sodium Hydroxide Molar Mass
Calculate the molarity of the solution. 50 g of sodium hydroxide molar mass 40 g m o l 1 is dissolved in little quantity of water and the solution is diluted up to 100 ml.
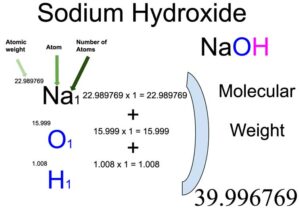
Sodium Hydroxide Naoh Molecular Weight Calculation Laboratory Notes
Sodium Hydroxide molecular weight.

. Ad Products empowering scientists at every stage helping to deliver scientific breakthroughs. Firstly 40 NaOH implies 40 g NaOH in 100g solution. At 25C 77F or 29815K at standard atmospheric pressure.
NaOH is a strong base and very caustic. Molar mass of NaOH The atomic mass of Na The atomic mass of O The atomic mass of H 22. Ad Browse discover thousands of brands.
2NO3 - --- NO2 O2 2e. The molecular mass of sodium hydroxide is 40. Molar mass The atomic mass of element number of atoms given in subscript.
50 by mass ww Density of 50 ww Sodium Hydroxide solution. Convert grams Sodium Hydroxide to moles or moles Sodium Hydroxide to grams. So its molar mass is 22989159991007939996 gmol to 5 sig figs.
Free easy returns on millions of items. Concentration of Sodium Hydroxide solution. Leading life science supplier for your research development or production needs.
This the same as multiplying by the reciprocal of 40 gmol. In Imperial or US customary measurement system the density is equal to 132972 pound per cubic foot lbft³. Sodium hydroxide weighs 213 gram per cubic centimeter or 2 130 kilogram per cubic meter ie.
Of moles Given mass Molar mass 4040 1 mol Now assuming solvent to be water since it isnt specified density of water 1gmL Also. Molar mass of NaOH Sodium Hydroxide is 3999711 gmol. The Na formed at the cathode reacts immediately with the strongly oxidizing nitrate ions giving sodium oxide and nitrogen you may also get some sodium nitrite.
Read customer reviews find best sellers. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Thus mass of NaOH 40g No.
The molar mass of any compound can be calculated by adding the atomic weight of the individual atoms involved in the chemical formula of the respective chemical compound. Sodium Hydroxide NaOH Molar Mass Molecular Weight. Hence the Molar mass of NaOH is 39997 gmol.
So its molar mass is 22989159991007939996 gmol to 5 sig figs. Formula for molar mass. Free shipping on qualified orders.
The molar mass of NaOH sodium hydroxide is. Click hereto get an answer to your question 246 g of sodium hydroxide molar mass 40 are dissolved in water and the solution is made to 100 cm3 in a volumetric flask. We should know the molecular or chemical formula of the respective compound.
I hope you have understood the short and simple calculation for finding the molar mass of NaOH. Keep in mind that nitrate ion is a strong oxidizer and hence any sodium formed will be destroyed immediately. Molar mass of NaOH 3999711 gmol.
Answer 1 of 10. Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH. 2299 15999 1008.
See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Molar mass of Sodium hydroxide NaOH. Sodium hydroxideA few things to consider when finding the molar mass for NaOH- make sure you have the co.
Density of sodium hydroxide is equal to 2 130 kgm³. Molar Mass of Hydroxide OH. The molar mass of OH Hydroxide is.
Density W e i g h t V o l u m e. If 1 litre of sodium hydroxide solution is prepared by dissolving 12 g of it then the molarity of the solution will be. 22989770 159994 100794 Percent composition by element.
Its melting point is 318 C 6044 F boiling point 1388 C 25304 F density 213 gcm3. NO3 - --- NO O2 e. The mass of 1 mole of a substance is called its molar mass.
So Molar mass of NaOH Molar mass of 1 Sodium Na atom Molar mass of 1 Oxygen O atom Molar mass of 1 Hydrogen H atom. Explanation of how to find the molar mass of NaOH. Mass percentage of the elements in the composition.
Molar mass of Sodium Hydroxide. Volume Weight Density. NaOH is a white crystal at room temperature.
Calculate the volume of 100 grams of Sodium Hydroxide solution. 99 g mol-1 1600 g mol-1 10079 g mol. - In the question it is asked to calculate the molar mass of the sodium hydroxide.
If the equation is arranged correctly the mass units g cancel out and leave moles as the unit.

Sodium Hydroxide Caustic Soda Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs

Quiz Worksheet Molar Mass Study Com

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube
No comments for "Sodium Hydroxide Molar Mass"
Post a Comment